The requirements for medical device registration in India with the CDSCO is explained in this blog. In brief, the CDSCO came into existence in 2006 and has since then implanted several rules and regulations for medical device imports into the country. Medical devices which have approval from GHTF countries can register in India through a straight forward document driven process.
Medical devices which require registration in India include spinal needles, cochlear implants, annuloplasty rings, trachestomy tubes, syringes and needle, dental implants, surgical sealants, heart valves, cardiac stents, orthopedic implants, endotracheal tubes, and catheters, among others. A detailed list of notified medical devices in India can be found here.
Contact us to learn more about the document requirements.
Medical Device Registration India Report:
Analysis of Medical Devices Registered in India since 2006
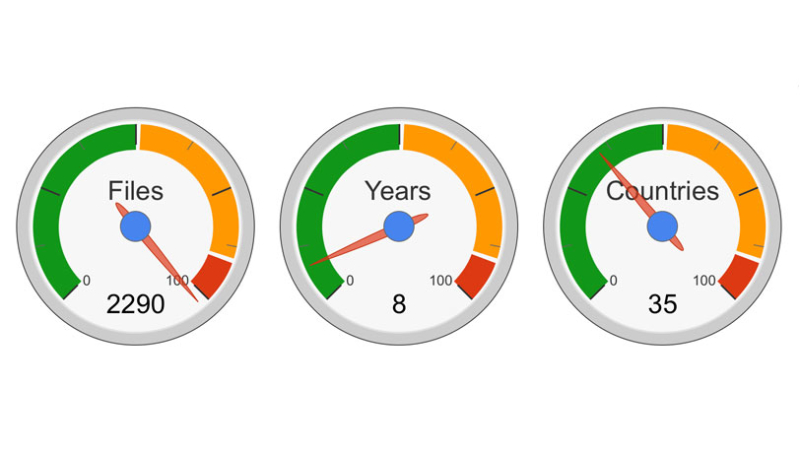
A total of 2290 files processed by the CDSCO since 2006 for the medical device registration in India process was analyzed by the Morulaa team of regulatory consultants. A brief summary of the report is provided below. Purchase an in-depth report.
Report 1: Area Wise Segmentation of Medical Devices in India
CDSCO data suggests that Cardiovascular, Orthopaedic implants, Vascular devices, Syringes and Needles, Wound Care and Surgical Dressings, Opthalmology, Spine, Urology and Dental are some of the sectors in which overseas manufacturers are aggressively bringing their medical devices into the Indian market. There has been an increase in demand for dental and wound care products in the past five years.
Report 2: Countries Exporting Medical Devices to India
Analysis of the CDSCO data shows the number of medical device companies registering in India from different parts of the world. From the US to Europe, Singapore to Australia, several companies have realized the potential of the Indian healthcare market and are registering their products with the Indian FDA. The above world map provides a heat image of the different countries which register their medical devices in India. The actual numbers can be checked by hovering the cursor over the region of interest. Below is a pie chart depicting the top ten countries which are actively involved in registering their medical devices with the CDSCO.
Report 3: Number of Registration Certificates Issued Per Year by CDSCO
For the medical device registration in India, there was an initial spike in the number of certificates issued by the CDSCO in 2006 – 2009 as this were the initial formative years and all medical devices which were being sold in the country had to submit their paper work for regulatory approval. The above figure shows that the CDSCO processes on an average 250 certificates per year in the past few years.
This blog article provides a brief of the files processed by the CDSCO since 2009. For an in-depth report just fill get in touch form and have our representative get in touch with you. A personalized report for your medical device can be developed by our team of experts with time lines, requirements etc.
The medical devices which Morulaa deals with include medical devices in Cardiology, Orthopaedics, Vascular, Syringes and Needles, Wound Care and Surgical Dressings, Opthalmology, Spine, Urology, Dental, ENT, Gastroenterology, Pulmonary, BloodStorage, Neurology, Aesthetics, Contraceptive, Surgery, Gynaecology, IVD and Oncology.